
Insights+: EMA Marketing Authorization of New Drugs in May 2023
Shots:
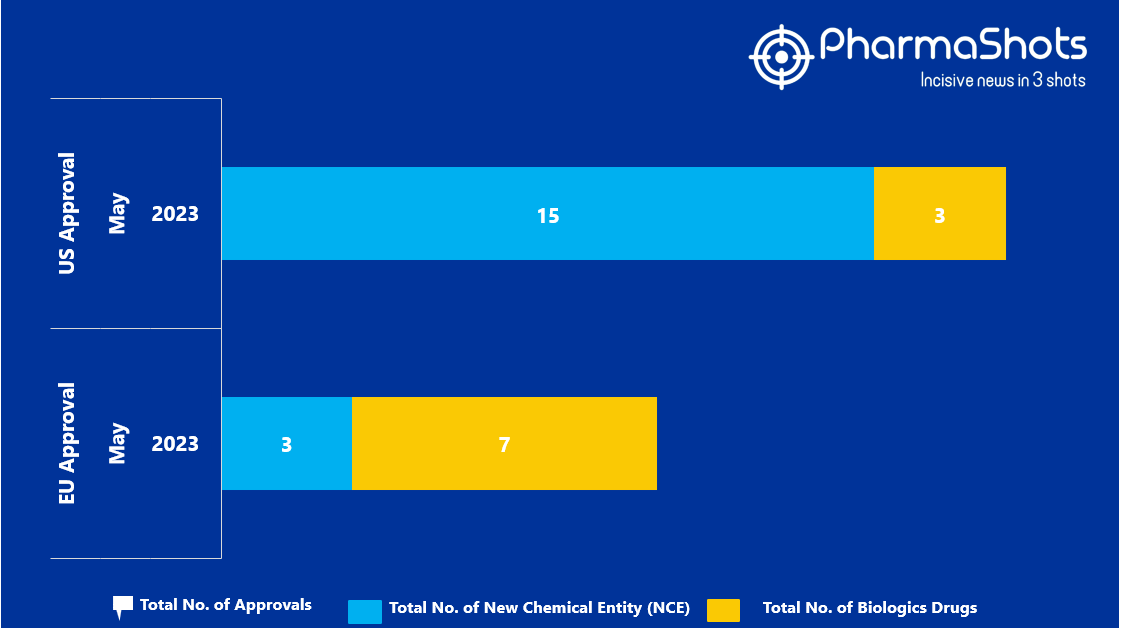
- The EMA approved 3 New Chemical Entity (NCE) and 7 Biologic Drugs in May 2023, leading to treatments for patients and advances in the healthcare industry
- In May 2023, the major highlights drugs were Breyanzi’s Approval for relapsed or refractory large B-cell lymphoma, Ultomiris (ravulizumab) for neuromyelitis optica spectrum disorder
- PharmaShots has compiled a list of a total of 10 new drugs approved by the EMA in May 2023
Breyanzi
Active ingredient: lisocabtagene maraleucel Approved: May 03, 2023
Company: BMS Disease: Large B-cell Lymphoma
- The EC has approved Breyanzi, a CD19-directed CAR T cell therapy for DLBCL, HGBCL, PMBCL & FL3B who relapsed within 12mos. from completion of or are refractory to 1L chemoimmunotherapy
- The approval was based on the P-III trial (TRANSFORM) evaluating Breyanzi vs SoC in 184 patients which showed improved EFS with a manageable and well-established safety profile, m-EFS (10.1 vs 2.3mos.) at the time of prespecified interim analysis with a median follow-up of 6.2mos.
- The primary analysis results were consistent with the interim analysis with a median follow-up of 17.5mos. & showed m-EFS (not reached vs 2.4mos.), CR (73.9% vs 43.5%) & m-PFS (not reached vs 6.2mos.). Breyanzi was approved in Japan for 2L treatment of r/r LBCL & in Japan, EU, Switzerland & Canada for r/r LBCL
PRX-102
Active ingredient: pegunigalsidase alfa Approved: May 05, 2023
Company: Chiesi Global Rare Diseases Disease: Fabry Disease
- The EC has granted marketing authorization to PRX-102 (pegunigalsidase alfa) in the EU for the treatment of adult patients with Fabry disease
- The EC authorization was based on the results from a comprehensive clinical development program in +140 ERT-naïve and ERT-experienced patients with ~7.5yrs. of treatment, incl. a head-to-head trial that met its 1EPs & showed non-inferior efficacy to agalsidase beta in controlling kidney disease as evaluated by eGFR decline
- PRX–102, a PEGylated enzyme replacement therapy (ERT) has been shown to have a circulatory half-life of ~80hrs. in clinical studies. The therapy is currently under the US FDA's review
Hepcludex
Active ingredient: bulevirtide Approved: May 05, 2023
Company: Gilead Disease: Hepatitis Delta Virus
- The EMA’s CHMP has adopted a positive opinion recommending the full marketing authorization for Hepcludex in adults with chronic HDV & compensated liver disease
- The opinion was based on P-III (MYR301) 48wk. study evaluating bulevirtide in 150 patients, showed a positive impact of bulevirtide on PROs with a greater combined virological & biochemical response (45% & 48%) over patients who had not received antiviral treatment (2%). The safety profile was consistent with prior reports with no patients having AE leading to discontinuation of bulevirtide
- Hepcludex will be the only approved treatment for HDV in the EU if granted by the EC. The company also works with multiple regulatory authorities on marketing applications for bulevirtide in other parts of the world
Tibsovo
Active ingredient: ivosidenib Approved: May 11, 2023
Company: Servier Disease: IDH1-Mutated AML and Cholangiocarcinoma
- The EC has approved Tibsovo as a targeted therapy in 2 indications i.e., in combination with azacitidine for adult patients with newly diagnosed AML with IDH1 R132 mutation & as monotx. for LA or metastatic cholangiocarcinoma with an IDH1 R132 mutation
- The approval was based on the results from the P-III study (AGILE) in AML, published in the NEJM evaluating Tibsovo + azacitidine vs PBO + azacytidine which showed an improvement in EFS & OS, m-OS (24.0 vs 7.9mos.) & 2EPs incl. CR rate, OS, CRh rate & ORR
- The approval in cholangiocarcinoma was based on the P-III trial (ClarIDHy) which showed an improvement in 1EPs of PFS, m-PFS (2.7 vs 1.4mos.), 32% vs 22% remained free of progression or death @6 & 12mos.Servier, Tibsovo, ivosidenib, IDH1-Mutated, Acute Myeloid Leukemia, Cholangiocarcinoma, Regulatory, EC, Approval
Ultomiris
Active ingredient: ravulizumab Approved: May 11, 2023
Company: AstraZeneca Disease: Neuromyelitis Optica Spectrum Disorder
- The EC has approved Ultomiris (C5 complement inhibitor) for adult patients with anti-aquaporin-4 (AQP4) Ab+ NMOSD
- The approval was based on the results from the P-III trial (CHAMPION-NMOSD) published in the Annals of Neurology evaluating Ultomiris in 58 patients across North America, the EU, Asia-Pacific & Japan which showed that Ultomiris met the 1EPs of time to first on-trial relapse
- Zero relapses were reported in patients with a median treatment duration of 73wks. (98.6% in relapse risk reduction) and continuing through a median duration of 90wks. & the safety & tolerability was consistent with prior studies and real-world use with no new safety signals. The regulatory submissions are under review with multiple health authorities, incl. in the US & Japan
6. Sobi Reports EMA’s Validation of MAA for Efanesoctocog Alfa to Treat Haemophilia A
Altuviiio
Active ingredient: Efanesoctocog Alfa Approved: May 19, 2023
Company: Sobi Disease: Haemophilia A
- Efanesoctocog alfa's MAA has been accepted & validated by the EMA for haemophilia A of all ages. The application was based on the P-III study (XTEND-1) & (XTEND-Kids) paediatric study evaluating efanesoctocog alfa (qw) in 159 & 74 patients aged ≥12yrs. & <12yrs.
- The (XTEND-1) study met 1EPs & EPs and showed prevention of bleeds & superior bleed protection over prior prophylaxis along with improvements in physical health, pain & joint health. In (XTEND-Kids) paediatric study, the trial met 1EPs with no factor VIII inhibitors detected confirming the safety profile of efanesoctocog alfa
- Efanesoctocog alfa, a novel & investigational recombinant factor VIII therapy was approved in the US as ALTUVIIIO in 2023
7. Gilead Receives EMA’s CHMP Positive Opinion to Extend the Use of Veklury (remdesivir) for COVID-19
Veklury
Active ingredient: remdesivir Approved: May 26, 2023
Company: Gilead Disease: COVID-19
- The EMA’s CHMP has granted a positive opinion for the use of Veklury (remdesivir) in COVID-19 patients with sev. renal impairment, incl. those on dialysis
- The positive opinion was based on the results from a P-I PK study (GS-US-540-9015) as well as results from the P-III trial (REDPINE) evaluating the safety of Veklury in a ratio (2:1) in 243 hospitalized adult patients hospitalized with COVID-19 with sev. renal impairment. In both trials, no new safety signals and no additional adverse reactions to Veklury were observed
- If Veklury was granted by the EC, it will become the first authorized antiviral treatment across all stages of renal disease. Veklury was approved in 50+ countries globally
Opdivo
Active ingredient: nivolumab Approved: May 29, 2023
Company: BMS Disease: Non-Small Cell Lung Cancer
- The EMA’s CHMP adopted the positive opinion recommending the approval of Opdivo + Pt-based CT for resectable NSCLC who are at a high risk of recurrence in adult patients with tumor cell PD-L1 expression ≥1%
- The opinion was based on the P-III trial (CheckMate -816) evaluating Opdivo (360mg, q3w) + CT vs CT alone which showed an improvement in EFS & pCR with 3 cycles of Opdivo + CT vs CT alone
- The safety profile was consistent with prior reported studies while the 3yr. data showed a durable clinical benefit. The combination therapy was approved for resectable NSCLC regardless of PD-L1 expression levels in 21 countries, incl. the US, Japan & China, additional regulatory applications are under review by global health authorities
Ztalmy
Active ingredient: ganaxolone Approved: May 30, 2023
Company: Marinus Pharmaceuticals Disease: Seizures
- The EMA’s CHMP has adopted a positive opinion recommending the approval of Ztalmy oral suspension for epileptic seizures associated with CDD in patients aged 2-17yrs.
- The application was based on the P-III (Marigold) trial evaluating Ztalmy vs PBO in 101 patients. The trial met its 1EPs & showed a median 30.7% vs 6.9% reduction in 28-day major motor seizure frequency. In the (Marigold) OLE study, a median 49.6% reduction in major motor seizure frequency for 12mos. & demonstrated efficacy, safety & tolerability
- The EC’s final decision is expected within 67 days of receipt of the CHMP opinion & will be valid to all 27 EU member states, Iceland, Norway & Liechtenstein. If Ztalmy is approved, it will be 1st treatment in the EU & will be commercialized by Orion
Sogroya
Active ingredient: somapacitan Approved: May 31, 2023
Company: Novo Nordisk Disease: Growth Hormone Deficiency
- The EMA’s CHMP has adopted a positive opinion recommending Sogroya (qw) for the replacement of endogenous GH in children aged ≥3yrs. & adolescents with growth failure due to growth hormone deficiency
- The opinion emerged following the data from the P-III study (REAL4) evaluating Sogroya (0.16mg/kg/week, SC) vs Norditropin (0.034 mg/kg/day, SC) in a ratio (2:1) in 200 treatments naïve, prepubertal children which showed a similar growth as children treated with Norditropin
- Annualized height (11.2 vs 11.7cm/yr.) with no statistical difference, was well tolerated with a similar safety & tolerability profile to the well-known profile of Norditropin. The EC’s final decision on the marketing authorization is expected in the coming months
Related Post: Insights+: EMA Marketing Authorization of New Drugs in April 2023
Note: Hepcludex, Veklury, Opdivo, Ztalmy & Sogroya received EMA’s CHMP Positive Opinion & EC’s Marketing Authorization for PRX-102 while EMA’s Validation of MAA for Efanesoctocog Alfa
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














